Inflammation in 3D
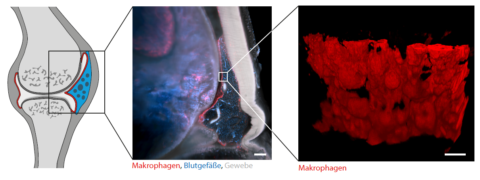
The exact causes of chronic inflammatory joint disease such as rheumatoid arthritis are still poorly understood. A team of researchers from the University Hospital Erlangen and the University Erlangen-Nuremberg has now chosen a new approach to better understand the underlying mechanisms. Stephan Culemann and Dr. Anika Grüneboom from the Department of Internal Medicine 3 – Rheumatology and Immunology – succeeded for the first time in making inflamed joints transparent and thus transparent to light with the aid of a specially developed technique. Subsequently, they examined these using three-dimensional molecular imaging. They gained surprising insights into how our immune system works. Among other things, it was shown that healthy joints are surrounded by a constantly self-renewing membrane of anti-inflammatory immune cells (macrophages). While this barrier of macrophages usually protects our joints from possible attacks of our own immune system, rheumatoid arthritis leads to a failure of this protective mechanism and thus to a sudden immigration of incorrectly activated immune cells, which ultimately cause joint inflammation and destruction. They have published their results in the renowned magazine “Nature “*.
A functioning immune system is essential for the survival of pathogens such as viruses and bacteria. In the context of chronic inflammatory autoimmune diseases such as rheumatoid arthritis or multiple sclerosis, however, a failed (auto-)immune reaction occurs, leading to inflammation and destruction of the body’s own organs. Through the combined use of new techniques such as 3-dimensional light sheet microscopy and single cell sequencing, the scientists have now been able to contribute key components to a better understanding of these diseases. Stephan Culemann and Dr. Anika Grüneboom from the Department of Internal Medicine 3 – Rheumatology and Immunology (Director: Prof. Dr. Georg Schett) succeeded for the first time in understanding the inflammatory process in rheumatoid arthritis on a cellular-molecular level in 3D. This approach led to the identification of membrane-like networks consisting of special resident immune cells (tissue macrophages) that wall in functional joints in healthy people and thus protect them. Molecular analyses showed that these macrophage membranes constantly renew themselves, similar to an intestinal epithelial layer. While macrophages have been suspected of contributing to joint inflammation, current analyses show that tissue macrophages actually form an important anti-inflammatory protective coat around the joint and can contain inflammatory reactions. “These insights into the functioning of the immune system not only shed new light on the complex distribution of tasks within the immune system, but also reveal new therapeutic options for chronic inflammatory diseases,” explains Prof. Dr. Gerhard Krönke, head of the study, which has just been published in the journal “Nature”.
* https://www.nature.com/articles/s41586-019-1471-1
Further Information:
Prof. Dr. Gerhard Krönke
Tel.: +49 (0)9131/85-34742
gerhard.kroenke@uk-erlangen.de
