Helicobacter Pylori induces gastric cancer
Stomach cancer is one of the five most deadly types of cancer: according to statistics from the World Health Organization (WHO), about 750,000 patients die of this disease every year. The main trigger is the bacterium Helicobacter pylori (H. pylori). There are currently no effective therapies for gastric cancer, and increasing antibiotic resistance makes it even more difficult to treat the infection. FAU researchers have now deciphered two mechanisms that lead to the development of gastric cancer by the bacterium. Their findings could contribute to the development of new therapeutic approaches. The scientists published the results in the renowned journal Cell host & microbe (doi: 10.1016/j. chom. 2017.09.005).
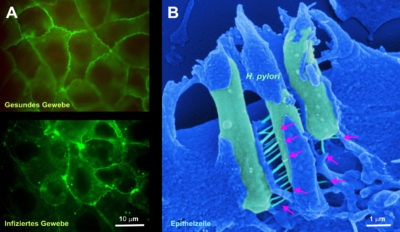
The team of international scientists led by Dr. Nicole Tegtmeyer from the FAU Department of Microbiology has investigated how bacteria destroy the protective layer in the stomach. This protective layer consists of epithelial cells lying close to each other, which protect us from gastric acid. The researchers have now discovered that the H. pylori bacteria use a secreted enzyme, the protease HtrA, as a weapon to break through this protective layer: HtrA cuts three proteins (Occludine, Claudin-8 and E-Cadherin) and creates a breakthrough in the epithelial cell layer. The H. pylori bacteria thus enter deeper, normally sterile tissue layers and cause further damage. This starts the development of gastric cancer.
However, this first step is followed by an even more dangerous one, as the scientists have found out. A needle-like appendix, known as a Type IV secretion system, is then activated and functions in the same way as a “molecular syringe”: it injects a bacterial toxin, the so-called CagA protein, at the bottom of the host cells via a receptor-dependent mechanism. The introduced CagA reprogrammes the host cell in such a way that cancer can develop. In addition, the protein influences the human immune system so that the bacteria are not recognized and thus not eliminated – a decisive way for the long-term survival of H. pylori in the human stomach.
New approach to gastric cancer therapy
Dr. Tegtmeyer assumes that these findings provide important new starting points for antibacterial therapy, as HtrA and CagA are excellently suited as new drug targets. The working group has already started testing specific inhibitors against HtrA. We hope that appropriate drugs will either completely prevent an infection or stop the CagA injection,”explains Tegtmeyer.
Die Veröffentlichung ist das Resultat einer mehrjährigen Forschungsarbeit mit Prof. Dr. Silja Wessler von der Universität Salzburg und Prof. Dr. Steffen Backert am Lehrstuhl für Mikrobiologie der FAU, die durch die Deutsche Forschungsgemeinschaft im Rahmen des Sonderforschungsbereichs „Schaltzellen zur Auflösung von Entzündung“ (SFB1181/TPA04 und Z02) und DFG TE 776 3-1 gefördert und in einer Kooperation mit weiteren Arbeitsgruppen aus Deutschland, Italien, Portugal und der Schweiz durchgeführt wurde.
Weitere Informationen:
Dr. Nicole Tegtmeyer
Tel.: 09131/85- 28988
nicole.tegtmeyer@fau.de
